
However, if consumption is less than recommended daily requirements, the body will supplement its energy sources by drawing upon stores of fat and the person will lose weight. If the average adult consumes more calories than the daily requirement, he or she will gain weight. In referring to the energy content of foods it is customary. The calorie used in chemistry and biochemistry is equal to 4.184 joules. These tabulations serve only as guides they cannot, of course, embrace all individual variations.įrom its daily intake of energy foods, the body uses only the amount it needs for energy purposes. calorie kal´o-re any of several units of heat defined as the amount of heat required to raise the temperature of 1 kilogram of water by 1 degree Celsius (1☌) at a specified temperature. Nutrition experts have computed daily calorie requirements in terms of age and other factors. Factors such as weight, age, activity, and metabolic rate determine a person's daily calorie requirement.
Calibre definition archive#
Subscribe to: Posts (Atom) Blog Archive 2013 (1) May (1) Medi-cal Definition About Me. What does CAL abbreviation stand for List of 527 best CAL meaning forms based on popularity. Posted by Unknown at 9:26 AM No comments: Email This BlogThis Share to Twitter Share to Facebook Share to Pinterest.
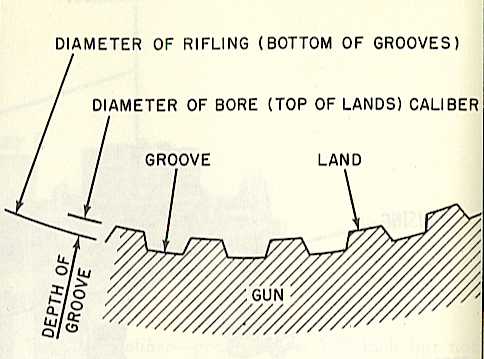
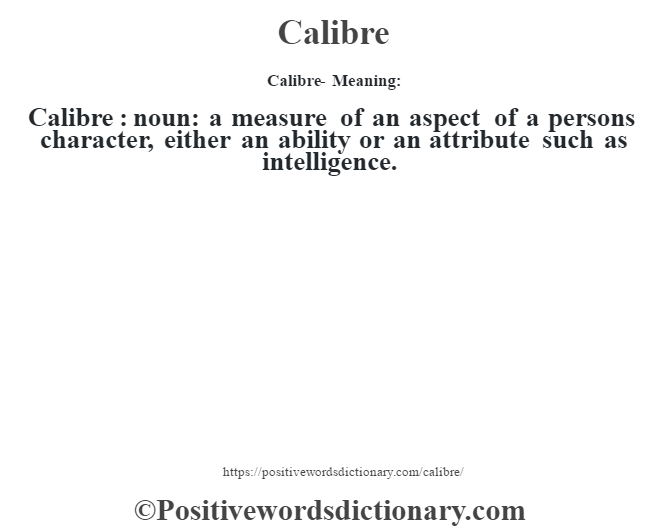
The amount of energy required for these chemical processes varies. medi-cal definition medi-cal definition click here. Digestive processes reduce food to usable “fuel,” which the body “burns” in the complex chemical reactions that sustain life. Every bodily process, including the building up of cells, motion of the muscles, and the maintenance of body temperature, requires energy, which the body derives from the food it consumes. they could ill afford to lose a man of his calibre. In referring to the energy content of foods it is customary to use the “large calorie,” which is equal to 1 kilocalorie (kcal). 1mass noun The quality of someones character or the level of their ability.
:max_bytes(150000):strip_icc()/cc1-56a553e75f9b58b7d0dc38bf.jpg)
Any of several units of heat defined as the amount of heat required to raise the temperature of 1 kilogram of water by 1 degree Celsius (1☌) at a specified temperature.


 0 kommentar(er)
0 kommentar(er)
